Microfluidics Technology:

We use microfluidics to create and manipulate nanoliter sized droplets to study protein crystallization and liquid crystalline phase transitions in virus solutions. A series of publications describe a device we are developing named the PhaseChip for the control and measurement of phase transitions in aqueous solutions. We have used the PhaseChip for two phase transition studies; a liquid-liquid transition in mixtures of salt and polymer and crystallization of a protein / polymer mixture where nucleation is enhanced by cyclically varying the protein's supersaturation.
The Brandeis Catalyst (pdf) has highlighted our research, as has Science and the New York Academy of Sciences.
Brandeis Microfluidics Facility
BMF
Open for Business!

Protein crystallization, currently a bottleneck in protein structure determination demands competing conditions: One on hand deep supersaturation is needed to stimulate nucleation of crystals; on the other hand low supersaturation is optimal for growth of crystals of quality suitable for x-ray diffraction and structural analysis. Added to this challenge is that experimentally not all thermodynamic variables are created equal. Temperature is the most "user friendly" as heat can pass through sealed chambers and change the temperature of the sample without having to open the chamber. In contrast, concentration is an exceedingly unfriendly variable, especially in the circumstances of protein crystallization where measuring out small quantities of viscous material is difficult and changing concentration requires opening sealed cells exposing the contents to the risk of evaporation or loss due to transfer. To address these problems, we have developed the Phase Chip, a microfluidic device that can formulate and store hundreds of nanoliter volume drops of protein solution in sealed wells.
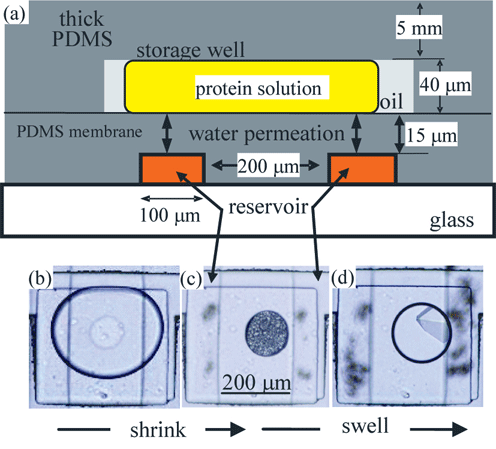
Each drop is separated by a thin semi-permeable membrane from a reservoir that is used to control the chemical potential of water in the protein drop as illustrated in Fig 1a. For proteins, where every drop is precious, manipulating concentration via permeation renders concentration as convenient a thermodynamic variable as temperature. Fig 1b shows the initial drop. In Fig 1c, after a concentrated salt solution is introduced into the reservoir the drop shrinks concentrating the solutes and driving the protein solution deep into supersaturation inducing the nucleation of many small crystals. In Fig 1d the salt concentration in the reservoir is reduced and the drop has swollen, lowering the supersaturation and raising the nucleation barrier. This causes the small crystals to melt and the large ones to grow, ultimately leading to the transformation of the small crystals into a single large crystal. This process, known as Ostwald ripening was described in the 19th century, but the Phase Chip promises to make this process practical for protein crystallization.
